1. From geospatial to spatial transcriptomics
Lambda Moses
dlu2@caltech.eduLior Pachter
lpachter@caltech.edu2022-07-27
Source:vignettes/vig1_intro.Rmd
vig1_intro.RmdAn analogy from geographical space
Data from bulk RNA-seq, single cell RNA-seq (scRNA-seq), and spatial transcriptomics all come from spatially organized tissues (except for blood), where such spatial organization plays a role in the properties and functions of the tissues. For this reason, a geographical analogy may be more adequate than the traditional analogy of the smoothie vs. fruit salad vs. tart.
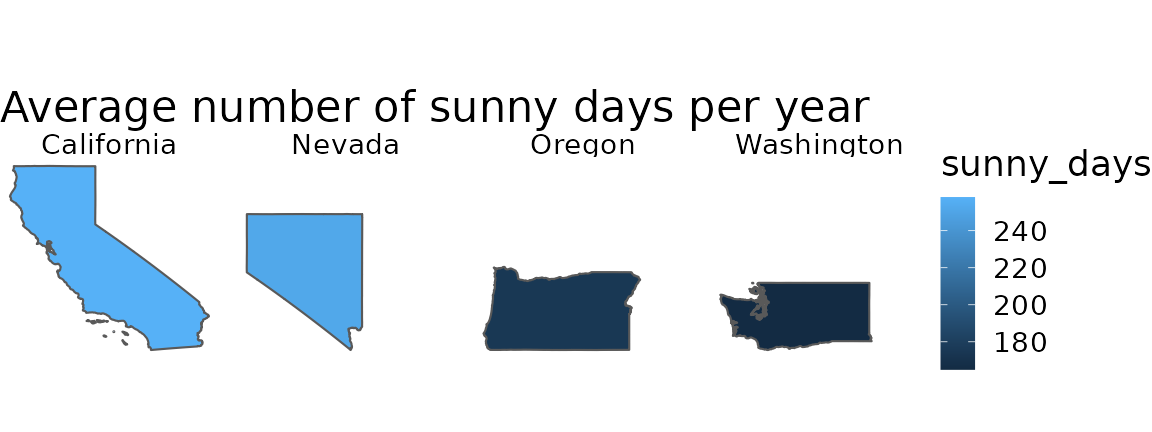
Many of you attending this conference in person have traveled to Seattle, and might explore Seattle after the talks. Bulk RNA-seq is an averaging assay, and in analogy, you could consider information averaged over Washington State. For example, on average, Washington State tends to have fewer sunny days per year than California. However, each state has diverse climate and weather, and tourists prepare for weather in individual cities rather than the state average.
Non-spatial scRNA-seq is like a list of tourist attractions in Seattle without spatial locations, where each tourist attraction is analogous to a cell. There’s the Space Needle, the Museum of Pop Culture, Chihuly Garden and Glass, Smith Tower, Pike Place, Museum of Flight, and etc. These tourist attractions can also be classified based on their characteristics, such as architectural style, function, and era of construction, analogous to gene expression. Dimension reduction can be performed on these numerous characteristics. Compared to the state averages, this would be of more interest to a tourist.

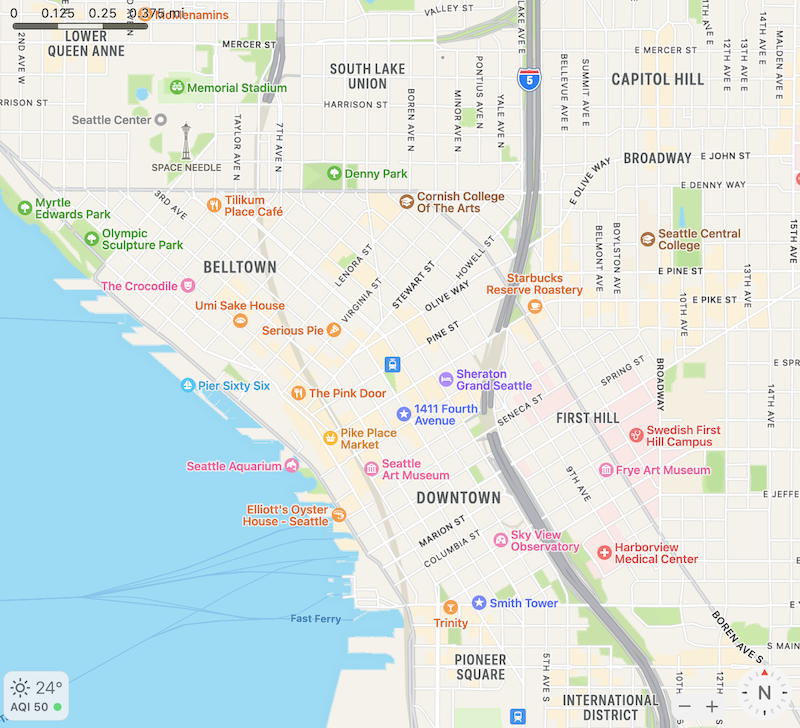
But how does one navigate to various tourist attractions? When you look at the map, you find that certain kinds of tourist attractions tend to cluster in space, such as the cluster of museums in the vicinity of Space Needle and the cluster of older buildings around Smith Tower. Different regions of Seattle with different vibes and functions are also annotated on the map. There are historical reasons that led to such spatial regions and clustering, such as the 1962 World’s Fair that gave rise to the Space Needle. Here we see how locating the tourist attractions in space point to a deeper understanding of the properties of Seattle. Spatial transcriptomics is like studying a map of Seattle.
Introduction to spatial transcriptomics data
Spatial transcriptomics helps us to make maps of the tissue, so just like in the geographical map, we may locate characteristics of cells in space and find spatial neighborhoods of cells. A working definition of “spatial transcriptomics” is any attempt to quantify the transcripts of more genes than can be done in one round of fluorescent in situ hybridization (FISH) while preserving the spatial context of the gene expression in the tissue. Since 2018, interest in spatial transcriptomics – in both data collection and data analysis – has grown drastically.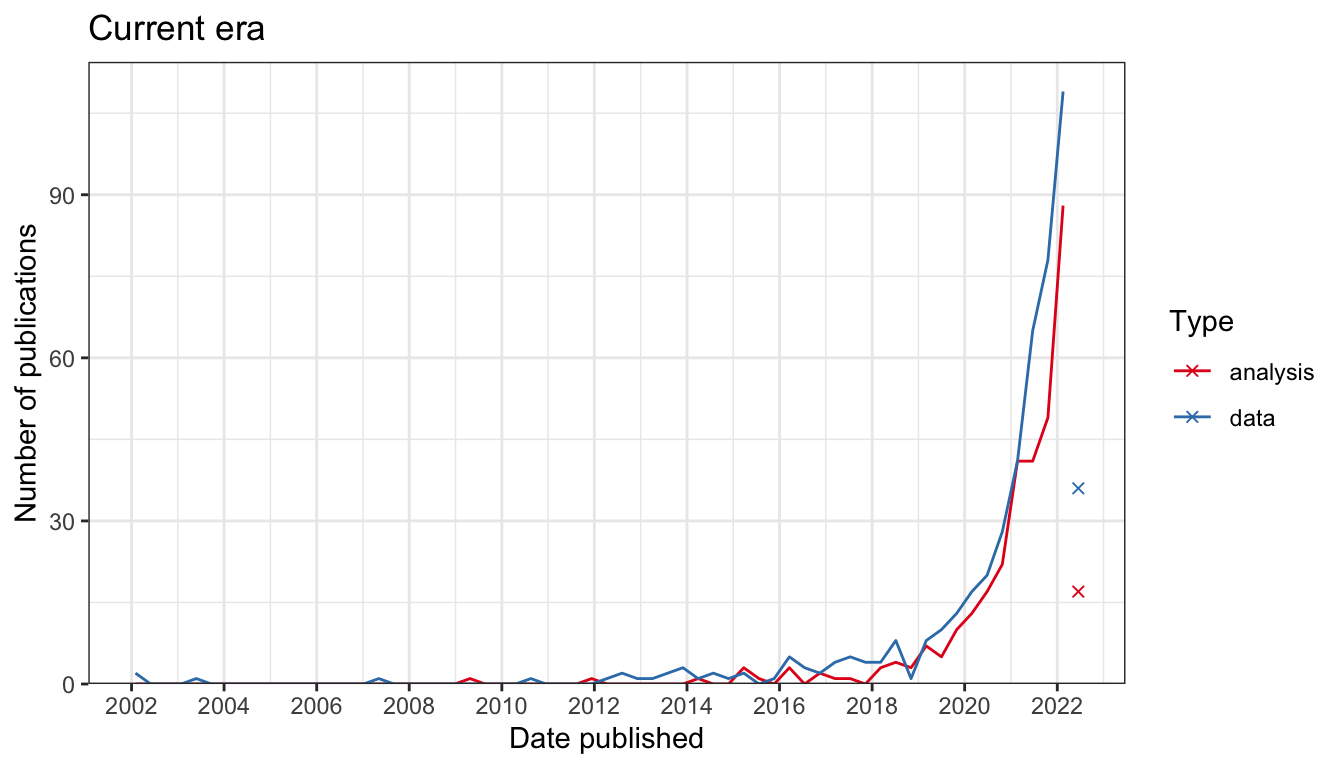
Number of publication over time for current era data collection and data analysis. Bin width is 120 days. The x-shaped points show the number of publications from the last bin, which is not yet full.
Based on how the spatial context is preserved, spatial transcriptomics data collection technologies fall into 5 categories, sorted by extent of current usage (plot showing number of publications per category), though there are gray areas:
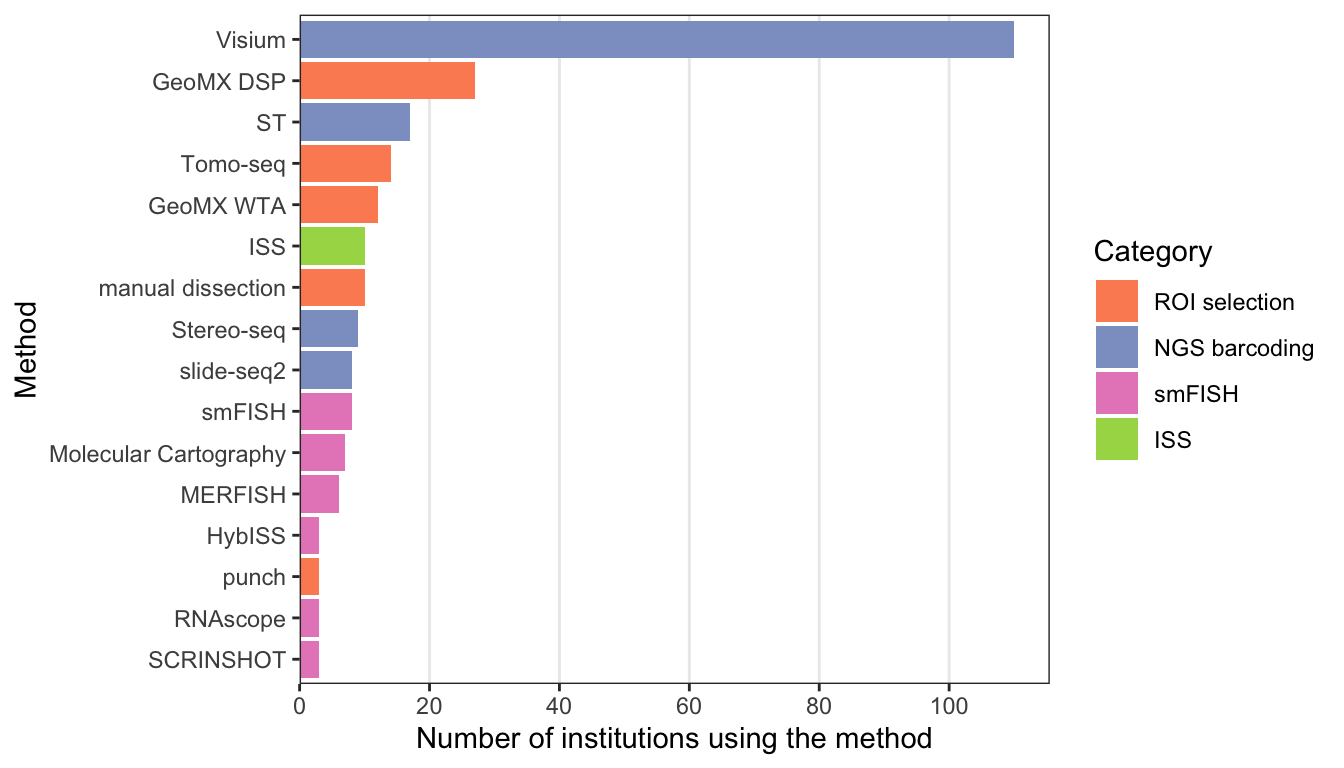
Techniques used by at least 3 institutions and the number of institutions that have used them.
- Next generation sequencing (NGS) with spatial barcoding, where spatial locations of the barcodes are known, such as 10X Visium, Slide-seq, and Stereo-seq. Many of these methods, such as the popular Visium, do not have single cell resolution. Hence cell type deconvolution is commonly performed.
- Region of interest (ROI) selection, where the act of specifying the ROIs itself defines the spatial locations, such as laser capture microdissection (LCM) followed by NGS and GeoMX DSP, where UV light is used to cleave probes only in the selected ROIs for quantification. The ROIs can also be sections in one spatial dimension, such as in Tomo-seq. These methods also generally don’t have single cell resolution.
- Single molecular FISH, where each detected transcript can be seen as a discrete puncta and counted, such as MERFISH, seqFISH, and Molecular Cartography. Combinatorial barcoding over multiple rounds – where fluorescent probes are hybridized to the transcripts or cDNAs and color or presence or absence of the probes are recorded and genes are identify by the sequence of colors or presence/absence – is used to quantify more genes than easily discernible colors simultaneously. Datasets from highly multiplexed smFISH are usually not genome wide, and only quantify a few hundred genes at a time, though these technologies have been used in an increasing number of cells per experiment.
- In situ sequencing (ISS), which is similar to smFISH as the transcripts are visualized as discrete puncta and counted. However, in ISS, the gene barcodes are determined by in situ sequencing, usually with sequencing by ligation, rather than with combinatorial barcoding. smFISH and ISS based data have single cell resolution.
- Spatial context is not directly recorded, but computationally reconstructed, such as by recording pairwise spatial adjacency of cDNAs as in DNA microscopy. In addition, the term “spatial” is sometimes used to refer to sub-cellular resolution, such as in APEX-seq, where transcripts are “localized” or organelles; since no spatial coordinates are recorded, packages in this workshop may not be relevant to these technologies.
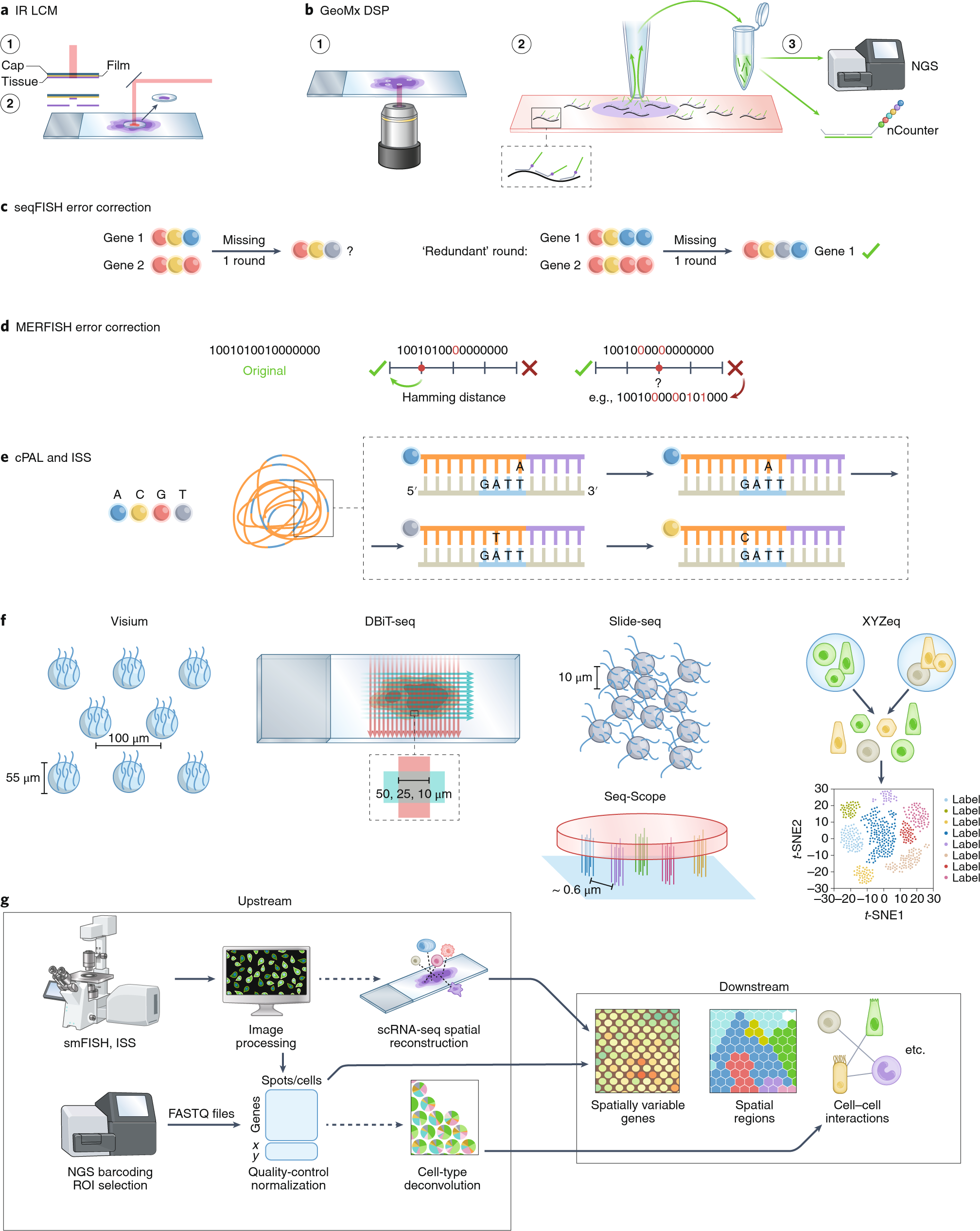
Data analysis methods written for spatial transcriptomics can be
broadly categorized as upstream and downtream. In upstream analysis, the
raw data is converted into more usable forms, such as getting the gene
count matrix from fastq files and cell type deconvolution of Visium
spots. Downstream analysis begins with the more usable form of data for
further biological inferences, such as finding spatially variable genes,
spatial regions informed by gene expression, and cell-cell interactions.
SpatialFeatureExperiment, as a way to represent data, is
more upstream, while Voyager, for exploratory spatial data
analysis (ESDA), is a little more downstream.
In the literature when spatial transcriptomics data is generated, it is often treated as non-spatial scRNA-seq data, and some data analysis methods for spatial transcriptomics data do not take the spatial information into account and are sometimes aimed at non-spatial data as well. For example, most cell type deconvolution methods don’t take into account spatial autocorrelation in cell type distribution, and deconvolution methods for bulk RNA-seq is sometimes used for Visium data. However, the spatial information presents many opportunities unavailable to scRNA-seq, and this workshop will explore some of these opportunities. Among the opporunities are:
- Identifying spatial regions characterized by gene expression of cell types, just like neighborhoods in geographical space. Many methods have been written for this purpose.
- Tissue and cellular morphology is available along side transcriptomics, and may be related to gene expression. This is explored a little in this workshop.
- Explore how gene expression and quality control (QC) metrics vary in
space, such as strength, length scale, and direction (anisotropy) of
spatial autocorrelation. This workshop explores strength and length
scale of spatial autocorrelation; anisotropy will be supported in a
later version of the
Voyagerpackage.
Geospatial data types and how they may relate to spatial transcriptomics
Opportunities presented by spatial data can be explored by leveraging
a vast tradition of tools made for geospatial data. Geospatial data
broadly fall into two types by representation: vector and raster. Vector
data represents the world as points, lines, and polygons, specified by
coordinates. For example, polygons would be specified by coordinates of
the vertices. Raster data are basically images, where each pixel has a
value, though unlike the typical RGB image, raster data can have many
different layers analogous to the 3 channels in RGB images. Raster is
common in remote sensing.
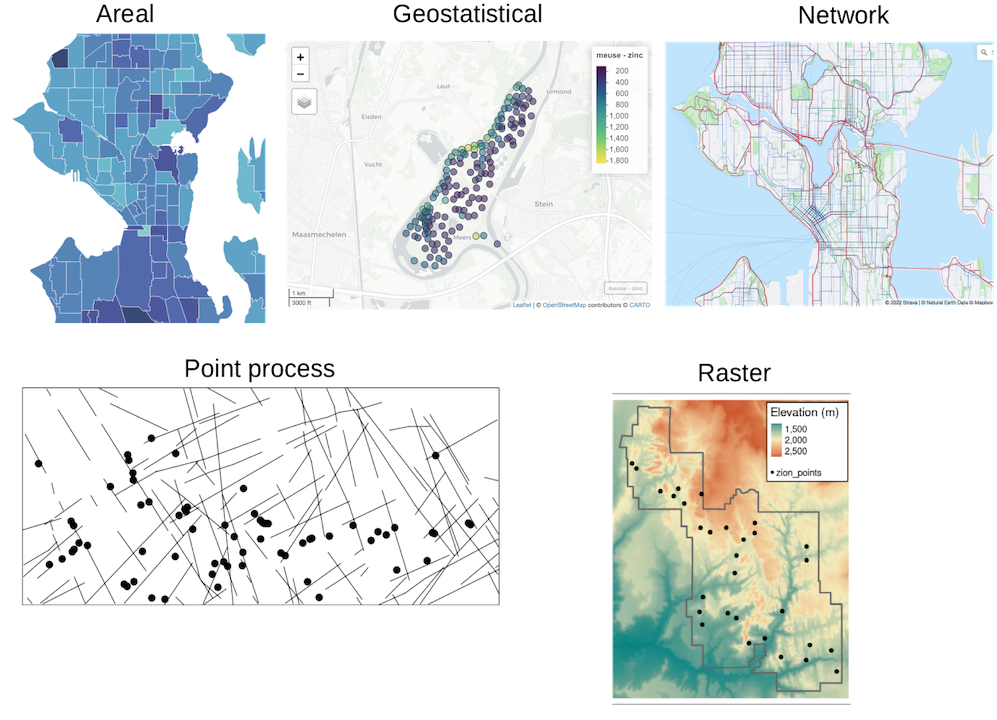
Vector data can be further classified by the processes generating the data:
- Areal, where values are for entire areas (e.g. cities, zip codes) rather than individuals. This is common in epidemiology to protect patients’ privacy.
- Geostatistical, where a spatial process (e.g. Gaussian process) is sampled at points in space, and the locations of those points are known and not of interest by themselves. Rather, the values are of interest. An example is measurements of pollutant concentration at points in space.
- Point process, where the locations of occurrence is of interest, such as where individuals of a species of plants occur. The locations themselves are analyzed to find if they are randomly distributed, clustered, or repelling each other, and if they are affected by covariates.
- When a phenomenon can only occur on a spatial network, such as roads and rivers. This can be a point process, such as locations of traffic incidents. This can also be analogous to raster data, such as Strava’s road/trail popularity heatmap, or areal when Strava’s heatmap is summarized over segments.
For each of these data types, there are already well-established
tools for data analysis. For example, sf represents vector
data and makes it behave like a data frame in R, spdep can
be used for spatial dependency of areal data, INLA can be
used for Bayesian spatial modeling, gstat is used for
interpolation with geostatistical data (kriging), spatstat
is used for point process data, sfnetworks for network
data, and spatstat supports point process analysis within a
network.
If we can relate these data types to spatial transcriptomics, then we can take advantage of these data analysis tools:
- Visium, LCM, and GeoMX DSP data aggregate transcript counts over areas. In addition, in smFISH and ISS data, the counts are also often aggregated over cells, and the cells are represented as polygons in space. Here such data is analogous the areal data, although unlike for cities and zip codes, the polygons may not be contiguous most of the time.
- If gene expression, or gene expression program, are imaged as spatial processes, and the cells or Visium spots are imagined as points, ignoring their area, then the data is analogous to geostatistical data. (Shall I mention that R package using kriging)
- Locations of transcript spots in smFISH and ISS based data, and locations of cells and cell types can be thought of as spatial point processes.
- Spatial networks seem less relevant to spatial transcriptomics. One potential use case is blood vessels. However, since spatial transcriptomics data is usually obtained from thin sections of the original 3D tissue, very little of the original 3D blood vessel network remains in the section.
- With the regular grids, Visium and 2016 Spatial Transcriptomics data may be seen as a low resolution raster.
Some geospatial data analysis methods have already been used in spatial transcriptomics, such as Gaussian process regression (which kriging is based on), Potts model, spatial point process analyses, conditional autoregressive models, and global and local variants of Moran’s I. (get the references) However, these sptial transcriptomics packages and data analysis in the literature don’t fully explore the opportunities given by the spatial information mentioned above, nor do they always take advantage of the existing software infrastructure originally built for geospatial data.
This workshop presents the packages
SpatialFeatureExperiment and Voyager, which
bring more of the existing vector geospatial data analysis tools to
spatial transcriptomics. SpatialFeatureExperiment (SFE)
implements a new S4 class extending SpatialExperiment with
sf data frames representing cell or Visium spot polygons,
geometries of other objects, anatomical regions, and tissue boundaries,
and spatial neighborhood graphs. Voyager to SFE is just
like scater, scran, and scuttle
to SingleCellExperiment, implementing basic ESDA using
well-established geospatial tools and plotting functions for geometries
and ESDA results. With sf, we focus on vector areal data
here, because of the popularity of Visium, and that although the
fluorsecent or H&E images are raster, vector features extracted from
the images, such as cell and nuclei segmentation, and transcript spots,
are more commonly used for downstream analyses.
Limitations and future directions
No analogy is perfect, and these are some limitations of the geospatial analogy that would call for data analysis methods specific to spatial transcriptomics:
- Biological tissues are usually originally 3D. Although most spatial
transcriptomics datasets come from thin sections, some datasets come
from thicker sections. However, the z dimension usually has much lower
resolution than x and y if present, and might need to be treated
differently. Geospatial tools and resources are very focused on 2D. If
3D support is present (e.g. 3D spatial point processes in
spatstat), the support may be limited. - Spatial transcriptomics datasets can get much larger than most
geospatial vector datasets, from thousands of genes and thousands to
millions of cells or spots. There should be more emphasis on
multivariate spatial analyses, which we plan to add to a later version
of
Voyager. On disk tools exist for geospatial data, but are not supported by SFE yet. - Unlike cities and natural landscapes, development and structure of some tissues (e.g. the cerebral cortex) are tightly regulated and similar across individuals.